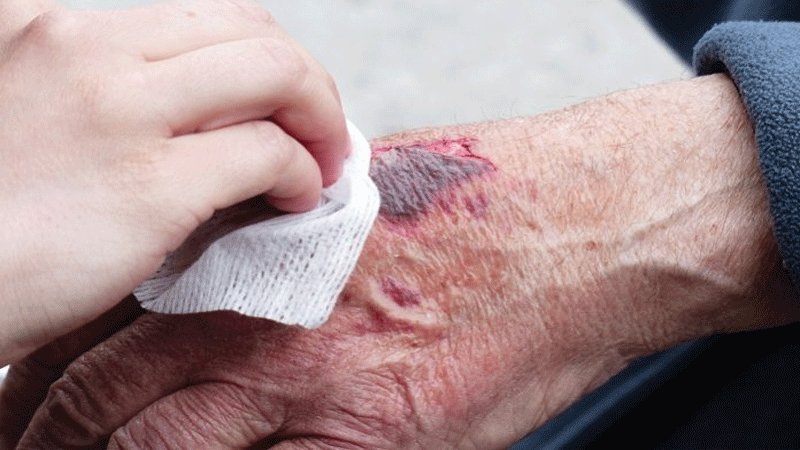
The first indigenously developed tissue engineering scaffold from mammalian organs, an animal-derived Class D Biomedical Device that can rapidly heal skin wounds at low-cost with minimum scarring, has received approval from the Indian Drugs Controller(IDC).
With this, the Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), an autonomous institution of the Department of Science and Technology (DST), became the first institution in the country to develop Class D medical devices that satisfy all statutory requirements of the Central Drugs Standard Control Organisation, Government of India.
The concept of using animal-derived materials as advanced wound care products is not new. However, indigenous technology was so far not available for fabricating quality products that satisfy the requirements of the Drugs Controller General. Therefore, such products were imported making them expensive.